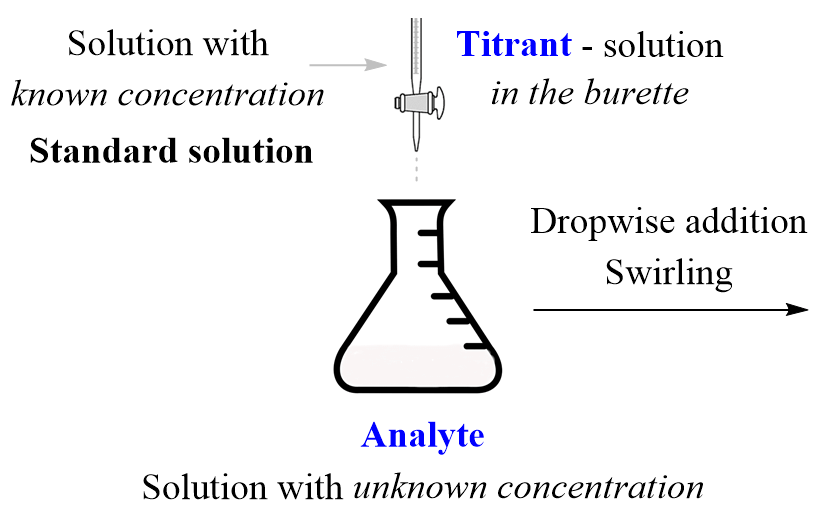
Acid Base Titration Is Also Known As . Explain what an indicator does. The analyte (titrand) is the solution with. Hna + noh− → na− + nh2o (13.5.1) It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. reactions in aqueous solutions. The reaction of an acid with a base to make a. Perform a titration calculation correctly. Titration is a common laboratory technique for determining the concentration of a solute.
from general.chemistrysteps.com
It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Hna + noh− → na− + nh2o (13.5.1) In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Perform a titration calculation correctly. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is a common laboratory technique for determining the concentration of a solute. The analyte (titrand) is the solution with. reactions in aqueous solutions. The reaction of an acid with a base to make a. Explain what an indicator does.
AcidBase Titrations Chemistry Steps
Acid Base Titration Is Also Known As The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with. reactions in aqueous solutions. Hna + noh− → na− + nh2o (13.5.1) It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Perform a titration calculation correctly. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Titration is a common laboratory technique for determining the concentration of a solute. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reaction of an acid with a base to make a. Explain what an indicator does.
From printablenemnserioeg.z22.web.core.windows.net
Titration Practical Questions And Answers Acid Base Titration Is Also Known As The analyte (titrand) is the solution with. The reaction of an acid with a base to make a. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is a common laboratory technique for determining the concentration of a solute. The concentration of a solution can be determined by. Acid Base Titration Is Also Known As.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Acid Base Titration Is Also Known As The concentration of a solution can be determined by knowing the acid and base dissociation constant. Perform a titration calculation correctly. Titration is a common laboratory technique for determining the concentration of a solute. Explain what an indicator does. The analyte (titrand) is the solution with. It is based on the neutralization reaction, where an acid and a base react. Acid Base Titration Is Also Known As.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration Is Also Known As It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Hna + noh− → na− + nh2o (13.5.1) Perform a titration calculation correctly. The reaction of an acid with a base to make a. Titration is a common laboratory technique for determining the concentration of a solute. The analyte (titrand). Acid Base Titration Is Also Known As.
From www.sciencephoto.com
Acidbase titration, 3 of 7 Stock Image C043/0045 Science Photo Acid Base Titration Is Also Known As The reaction of an acid with a base to make a. reactions in aqueous solutions. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Hna. Acid Base Titration Is Also Known As.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Acid Base Titration Is Also Known As The concentration of a solution can be determined by knowing the acid and base dissociation constant. Titration is a common laboratory technique for determining the concentration of a solute. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. The analyte (titrand) is the solution with. The. Acid Base Titration Is Also Known As.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Acid Base Titration Is Also Known As It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is a common laboratory technique for determining the concentration of a solute. reactions in aqueous solutions. The reaction of an acid with a base to make a. In its simplest form, titration is carried out by measuring the. Acid Base Titration Is Also Known As.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Acid Base Titration Is Also Known As The analyte (titrand) is the solution with. Titration is a common laboratory technique for determining the concentration of a solute. Explain what an indicator does. reactions in aqueous solutions. Hna + noh− → na− + nh2o (13.5.1) In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the. Acid Base Titration Is Also Known As.
From mungfali.com
Acid Base Titration Calculation Acid Base Titration Is Also Known As The analyte (titrand) is the solution with. Titration is a common laboratory technique for determining the concentration of a solute. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to. Acid Base Titration Is Also Known As.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Acid Base Titration Is Also Known As Perform a titration calculation correctly. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. The concentration of a solution can be determined by knowing the acid. Acid Base Titration Is Also Known As.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Is Also Known As Explain what an indicator does. reactions in aqueous solutions. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is a common laboratory technique for determining the concentration of a solute. The reaction of an acid with a base to make a. The analyte (titrand) is the solution. Acid Base Titration Is Also Known As.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Acid Base Titration Is Also Known As The reaction of an acid with a base to make a. Explain what an indicator does. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Hna + noh− → na− + nh2o (13.5.1) Perform a titration calculation correctly. reactions in aqueous solutions. In its simplest form, titration is carried out by measuring. Acid Base Titration Is Also Known As.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Acid Base Titration Is Also Known As Hna + noh− → na− + nh2o (13.5.1) In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with. The reaction of an acid with. Acid Base Titration Is Also Known As.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Acid Base Titration Is Also Known As The concentration of a solution can be determined by knowing the acid and base dissociation constant. Explain what an indicator does. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The reaction of an acid with a base to make a. Perform a titration calculation correctly. The analyte (titrand). Acid Base Titration Is Also Known As.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Acid Base Titration Is Also Known As Titration is a common laboratory technique for determining the concentration of a solute. The analyte (titrand) is the solution with. The reaction of an acid with a base to make a. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Explain what an indicator does. It. Acid Base Titration Is Also Known As.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Acid Base Titration Is Also Known As Hna + noh− → na− + nh2o (13.5.1) Explain what an indicator does. The reaction of an acid with a base to make a. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. It is based on the neutralization reaction, where an acid and a base. Acid Base Titration Is Also Known As.
From studylib.net
Titrations of Acids and Bases Acid Base Titration Is Also Known As The concentration of a solution can be determined by knowing the acid and base dissociation constant. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Titration. Acid Base Titration Is Also Known As.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Acid Base Titration Is Also Known As In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. reactions in aqueous solutions. Hna + noh− → na− + nh2o (13.5.1) The reaction of an acid with a base to make a. Titration is a common laboratory technique for determining the concentration of a solute.. Acid Base Titration Is Also Known As.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Acid Base Titration Is Also Known As Titration is a common laboratory technique for determining the concentration of a solute. Explain what an indicator does. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the. Acid Base Titration Is Also Known As.